INTRODUCTION
SYNTHIA™ Retrosynthesis software Step-by-Step Retrosynthetic Analyses are based on our proprietary algorithms, which use an expert-coded database of chemistry rules to find all possible disconnections for your target molecule. You may then select the reaction you like best and start a new search for the suggested precursors until you land on commercial or published starting materials. It is a hands-on approach, that allows you to navigate step-by-step through the synthesis, starting with the target molecule, and working iteratively backwards.
The database of chemistry rules contains transformations that you would find in organic chemistry textbooks or in the literature. These rules have been hand-coded by PhD level chemists and can account for incompatible functional groups in your reaction or recognize functional groups that need to be protected.
This guide offers a step-by-step approach to setting up a Shared Path Library analysis using the SYNTHIA™ Retrosynthesis software and explains how the results are presented. A preloaded example of a Shared Path Library analysis sis is available in your account for your reference.
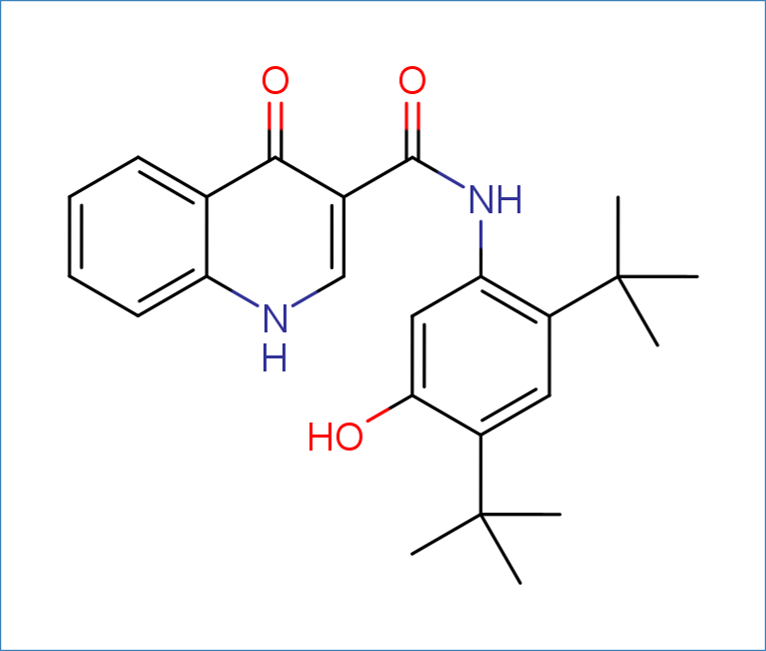
The molecule used throughout this manual is Ivacaftor, CAS number: 873054-44-5, SMILES string: CC(C)(C)c1cc(C(C)(C)C)c(NC(=O)c2cnc3ccccc3c2O)cc1O
.png)

