Lock Bonds as ‘Not Reaction Center’
This feature allows you to lock selected bonds as ‘Not Reaction Center’. SYNTHIA™ will then ignore these bonds for retrosynthetic cuts, and the selected bonds will be preserved with the synthesis.
For example, consider a search performed on a cyclohexanone derivative without using ‘Not Reaction Center’ (Pathway A), and another performed using this feature (Pathway B).
While Pathway A is a valid method for preparing this compound, you may decide that you do not want to build the cyclohexene ring via a Robinson annulation, but rather use building blocks in which the stereochemistry is already incorporated.
Pathway A
The top scoring pathway is a 3-step synthesis starting with an enantioselective allylation of alcohols, followed by an olefin metathesis, and an asymmetric Robinson annulation.
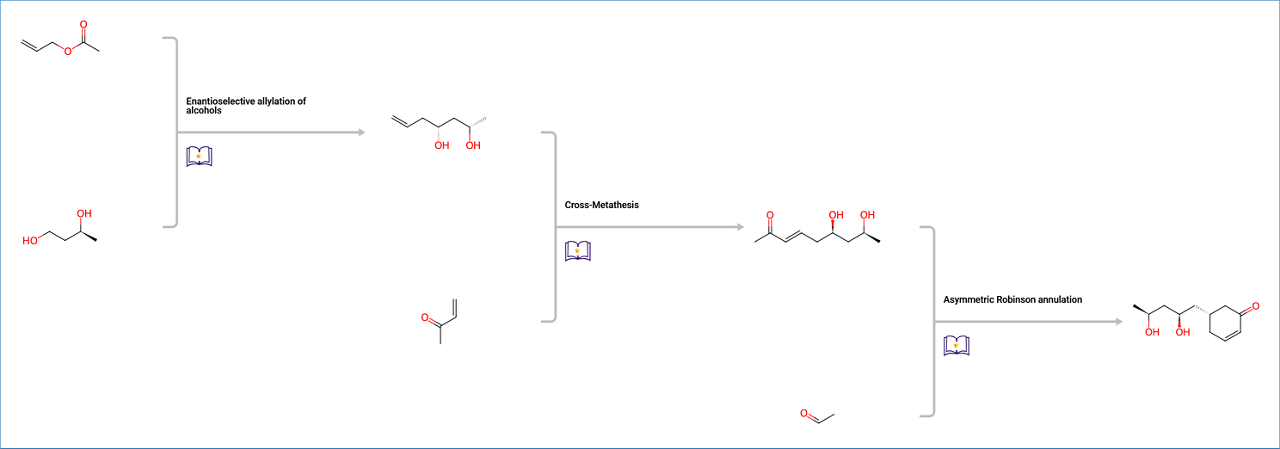
Pathway B
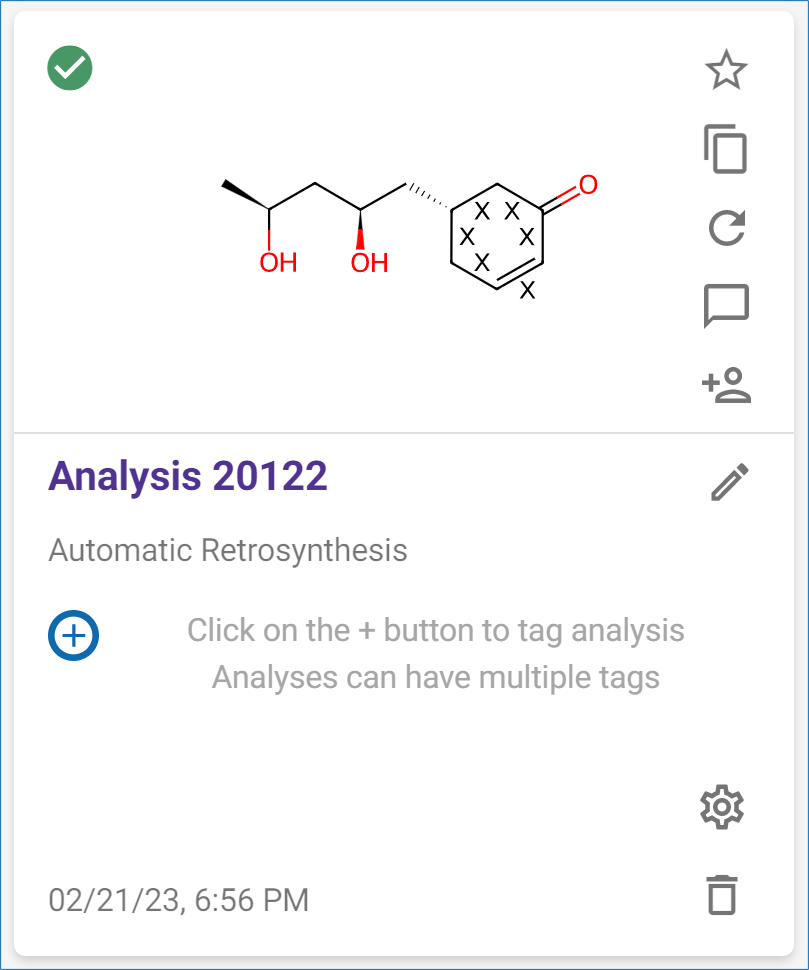
The top scoring pathway is a 3-step synthesis starting with an Appel reaction, ring opening of lactones with organolithium reagents, and asymmetric reduction. The cyclohexanone ring is integrated as a building block and preserved during the synthesis.
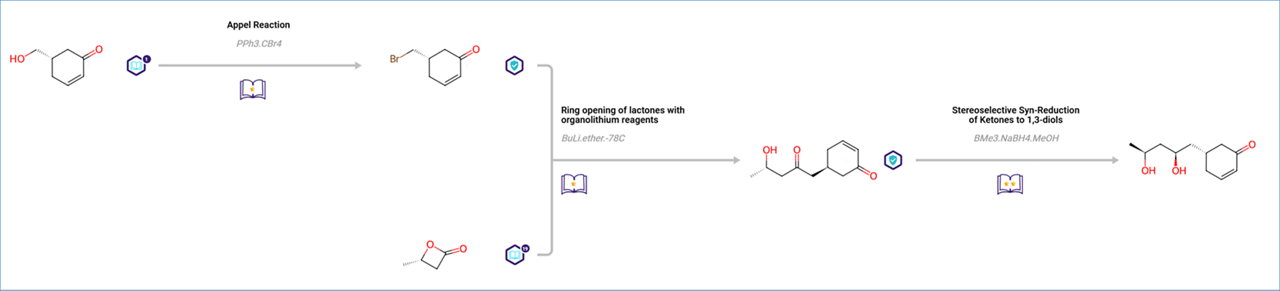
If you want to lock specific bonds in your target molecule, you may define them as ‘Not Reaction Center’ by following the steps below:
- Draw the desired structure in the molecule editor,
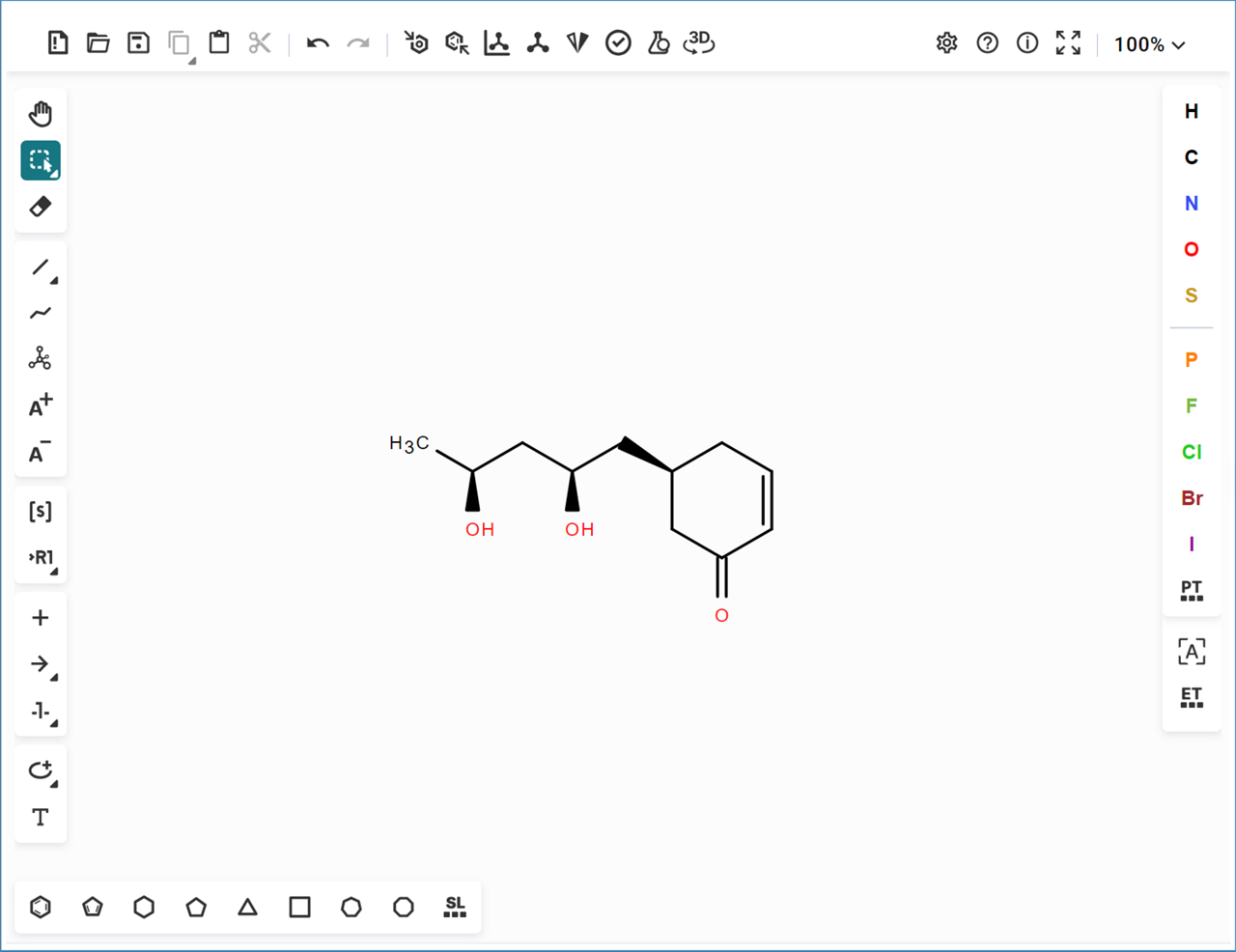
- Highlight the bonds that should be locked,
- The selection turns green,
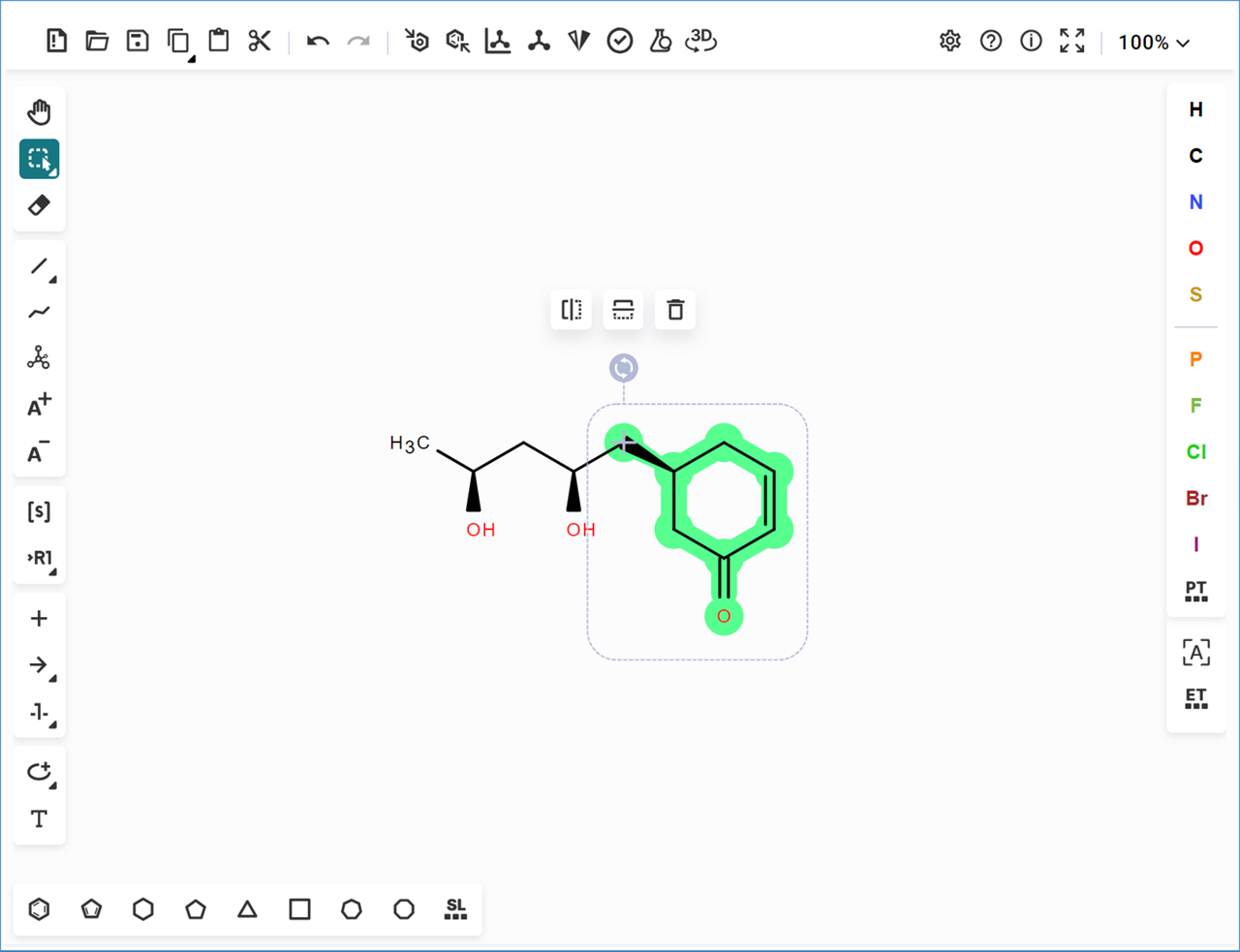
- Double click on one of the selected bonds to open the bond menu,
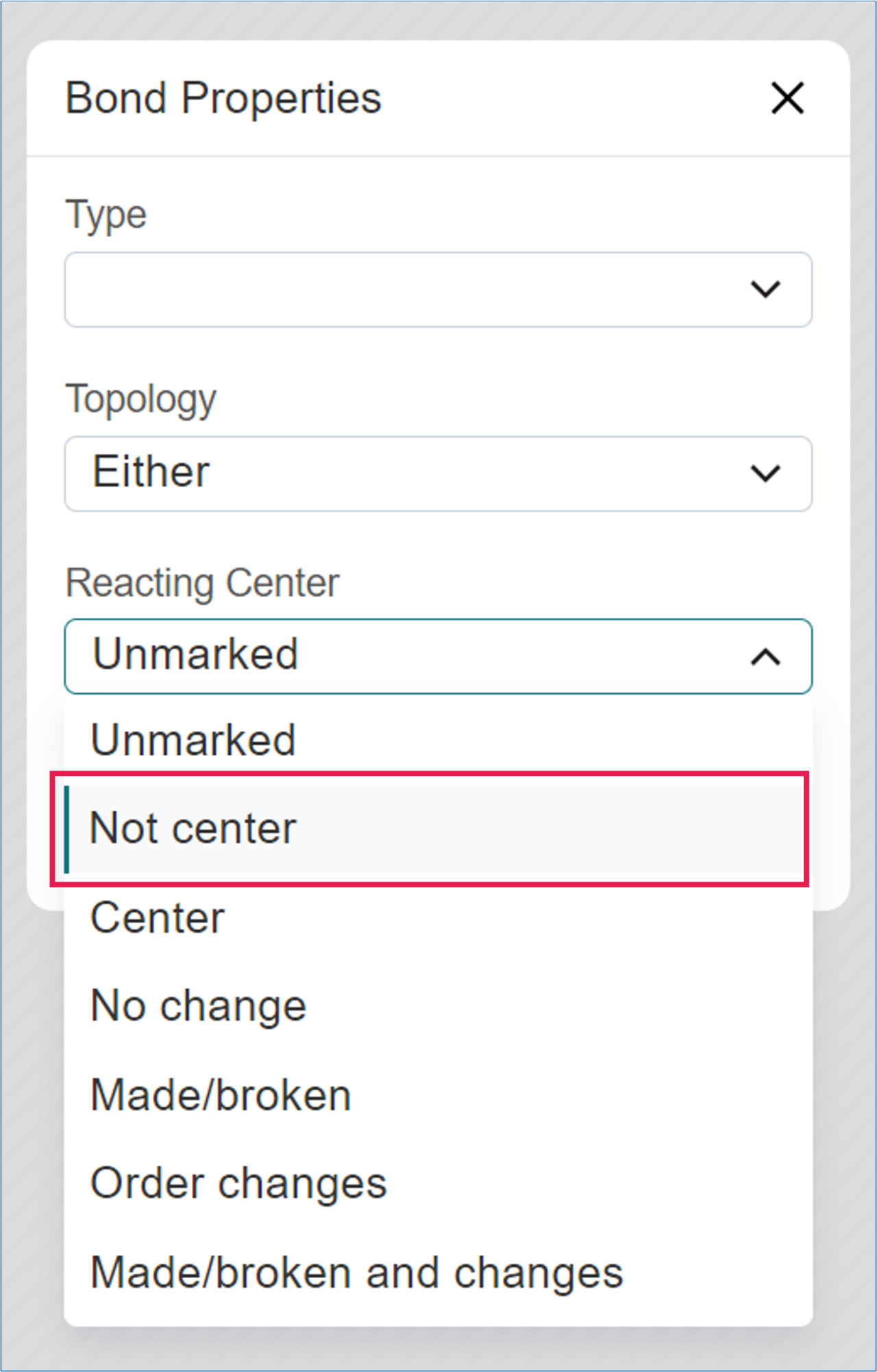
- Under ‘Reaction Center’, select ‘Not center’ and click ‘Apply’,
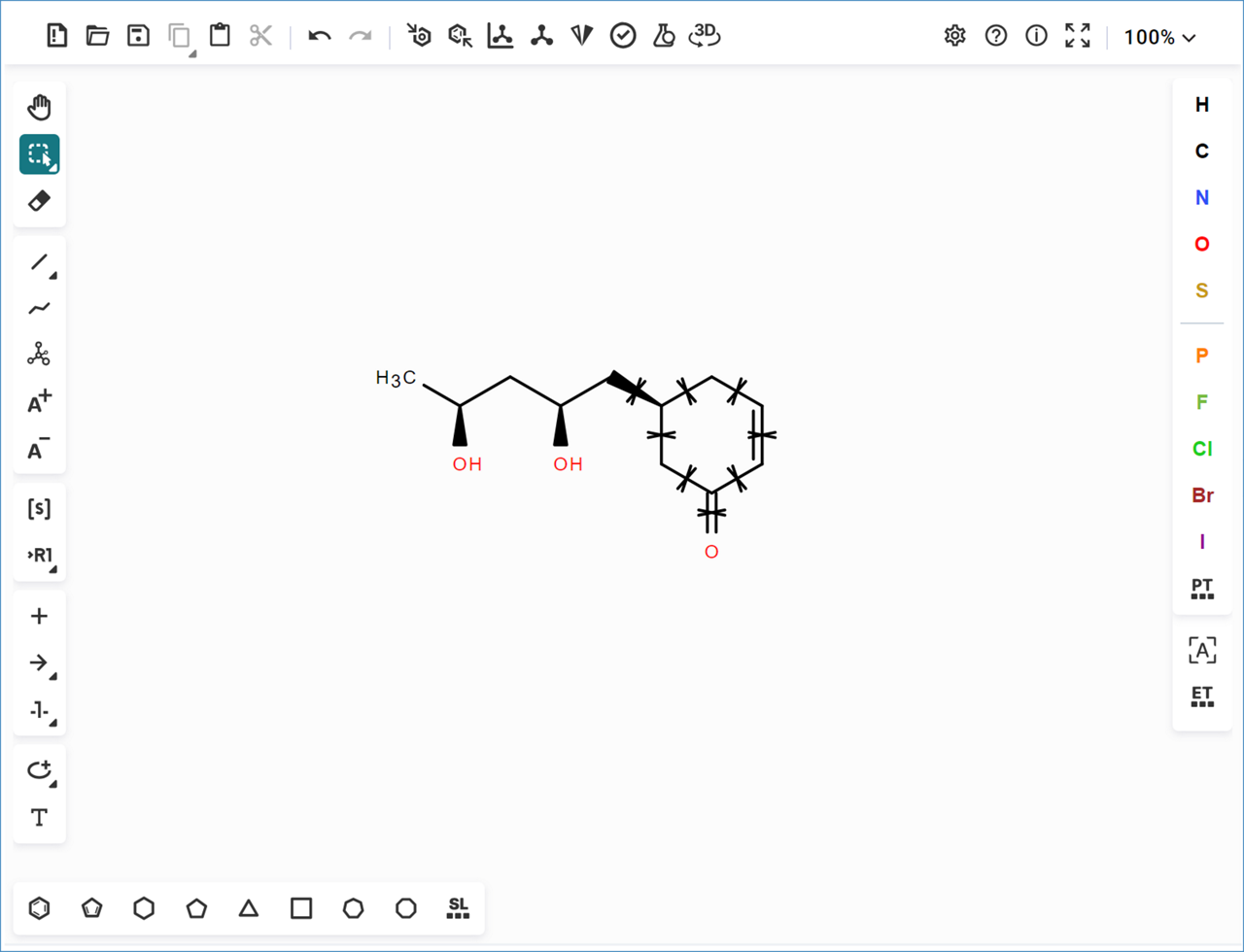
- The selected bonds show an ‘X’ through them, marking them as ‘Not a Reaction Center’,
- Select your analysis parameters,
- Start the analysis.
Note: The locked bonds will be marked with a ‘X’ next to them on the structure in the dashboard.
.png)

