Do you ever…
…wonder how SYNTHIA™ works out which reactions are suggested in your pathways?
SYNTHIA™ uses two types of reaction databases:
- Rule-Based Reactions
- These reaction rules are based on transformations found in organic chemistry textbooks or the literature.
- They suggest practical reactions to build your target molecule and are written in a general way, allowing them to apply to any molecule, including novel ones.
- Published Reactions
- Reactions that have been published in the literature.
You can select which databases SYNTHIA™ uses in the Analysis Configuration page under the Analysis Preferences tab in the Advanced section..
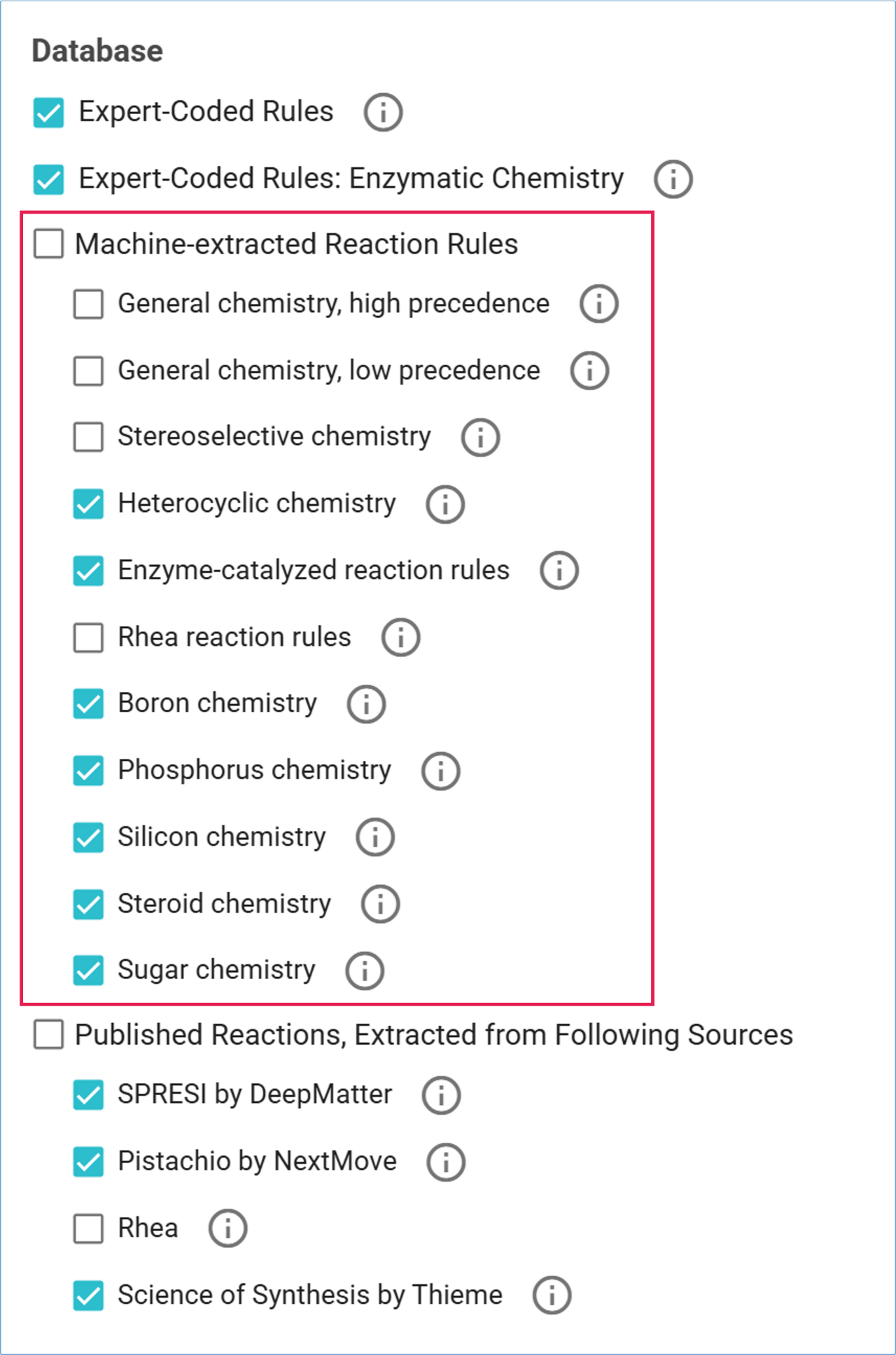
Expert-Coded Rules Database
SYNTHIA™ is built on an Expert-Coded Rules Database containing over 100,000 reaction rules. These rules:
- Are based on transformations found in organic chemistry textbooks and literature.
- Are written to apply to any molecule, including novel ones.
- Consider stereo- and regio-chemistry, protection requirements, reactivity conflicts, literature sources, and reaction conditions.
Enzymatic Reactions
SYNTHIA™ also includes a subset of expert-coded rules for enzymatic reactions, offering green alternatives to traditional chemistry.
By applying these rules to retrosynthetic disconnections, SYNTHIA™ suggests reactions that are possible based on known chemistry and compatible with the rest of the molecule structure.

Machine-Extracted Rules Databases
SYNTHIA™ includes five additional Machine-Extracted Rules Databases to supplement the expert-coded rules. These databases cover specialized chemistries and are ideal for unique structures, stereochemistry, complex aromatic systems, or unusual heterocycles.
Key Databases:
- General chemistry, high precedence: Reaction rules based on uncommon transformations that have a minimum of 10 published reaction examples.
- General chemistry, low precedence: Reaction rules based on rare transformations that have between 3 to 9 published reaction examples.
- Stereoselective chemistry: Reaction rules designed for stereoselective transformations.
- Heterocyclic chemistry: Reaction rules designed for the synthesis of aromatic heterocycles.
- Enzyme-catalyzed reaction rules: Reaction rules based on enzyme catalyzed reactions.
- Rhea reactions rules: Reaction rules extracted from the Rhea database of chemical and transport reactions of biological interest, which includes enzymatic reactions and transport reactions as well as reactions that occur spontaneously in biological systems, such as metabolic reactions.
- Boron chemistry: Reaction rules intended for introducing or substituting boron atoms.
- Phosphorus chemistry: Reaction rules intended for introducing or substituting phosphorus atoms.
- Silicon chemistry: Reaction rules intended for introducing or substituting silicon atoms.
- Steroid chemistry: Reaction rules based on commonly used chemistry for the syntheses of steroids.
- Sugar chemistry: Reaction rules based on commonly used chemistry for the syntheses of compounds containing sugars.
Important Notes:
- Machine-extracted rules may show unique chemistry, such as deprotection reactions or unusual heterocycle formations.
- Some rules may not include illustrative references and may lack detailed information on reaction conditions, reactivity conflicts, or protection requirements.
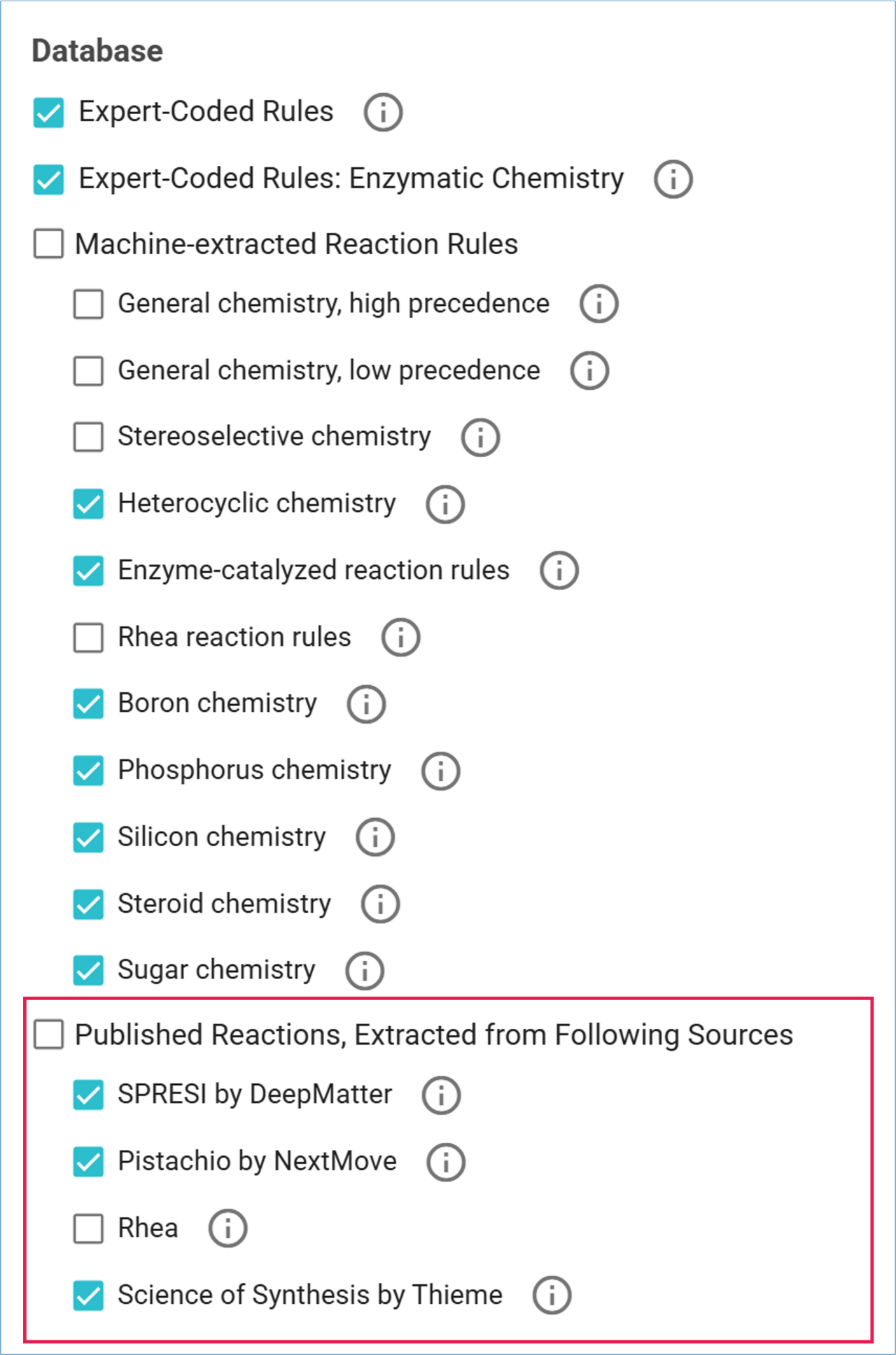
Published Reaction Databases
SYNTHIA™ also includes several databases of reactions published in the literature:
- SPRESI by DeepMatter
- Covers organic synthesis literature from 1974 to 2014.
- Includes 4.6 million chemical reactions abstracted from 700,000 references.
>> SPRESI by DeepMatter
- Pistachio by NextMove
- Includes over 10 million reactions sourced from patents filed with the USPTO, EPO, and WIPO, up to 2024.
- Rhea
- An expert-curated database of biochemical reactions, including enzymatic, transport, and spontaneous biological reactions.
>> Rhea
- Science of Synthesis
- Provides a critical review of synthetic methodologies developed from the early 1800s to date for organic and organometallic chemistry.
>> Science of Synthesis
Default Settings
By default, SYNTHIA™ enables:
- All Expert-Coded Rules.
- Machine-Extracted Rules for Heterocyclic Chemistry, Enzyme-catalyzed Reaction Rules, Boron Chemistry, Phosphorous Chemistry, Silicon Chemistry, Steroid Chemistry, an Sugar Chemistry.
- Published Reaction Databases: SPRESI, Pistachio, and Science of Synthesis.
Tips for Using Databases:
- Enable Stereoselective Chemistry to explore chiral bond formation.
- Enable Heterocyclic Chemistry for unusual heterocycles.
- Use General chemistry, high and low precedence to find diverse pathways or when results are limited.
- Disable the Pistachio database if you don’t want patent reactions in your results.
- Disable all published reaction databases to focus on predicted pathways.
Identifying Reactions in Results
- Path View: Published reactions display a book tag with three stars and a ‘100’ badge.
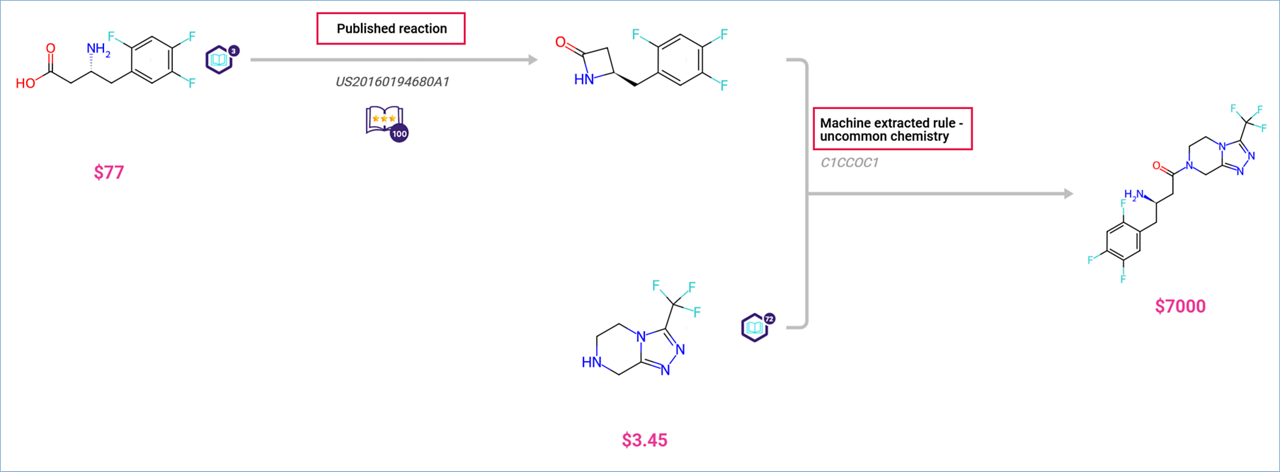
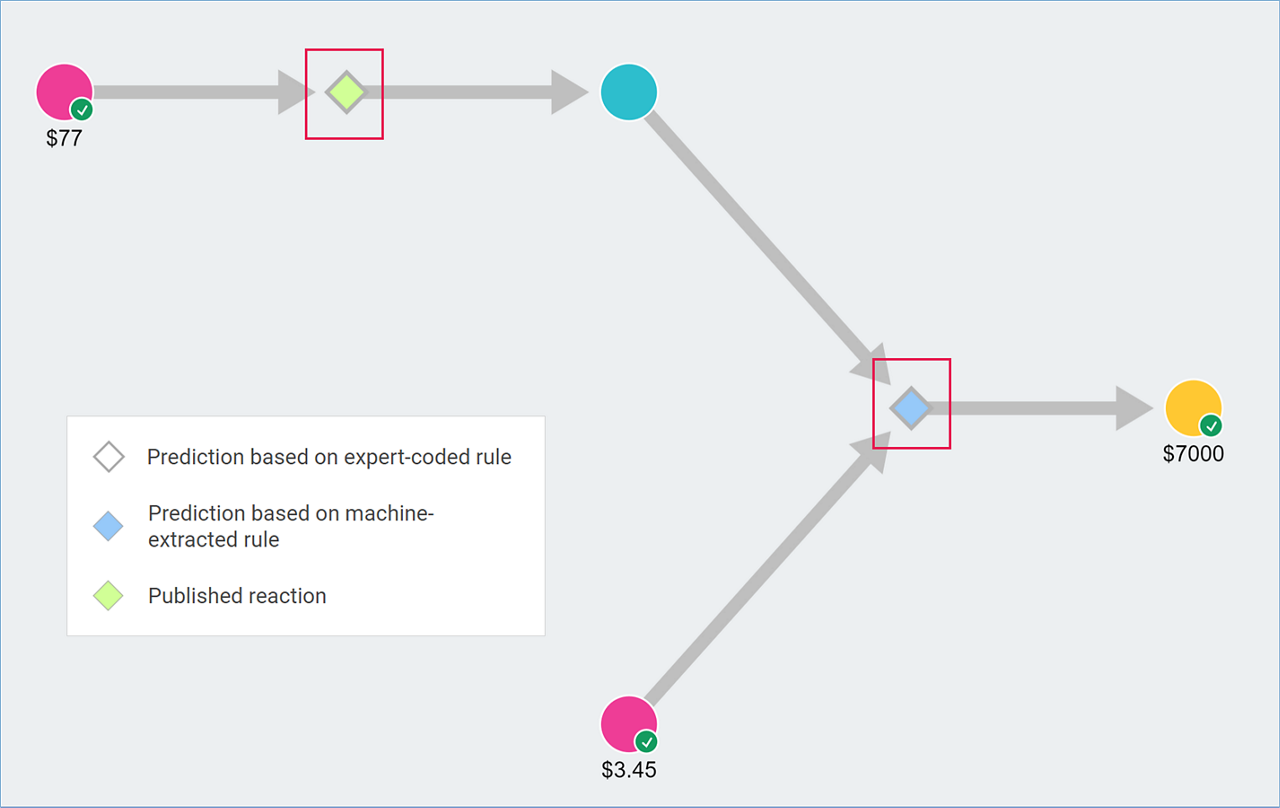
- Graph View: Reaction diamonds are colored:
- Blue for Machine-Extracted Rules.
- Green for Published Reactions.
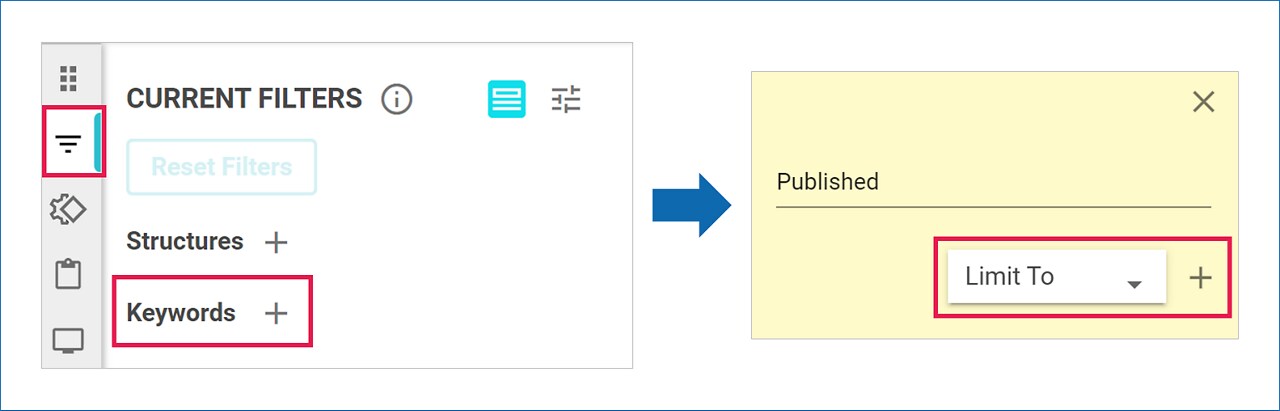
Filtering Results
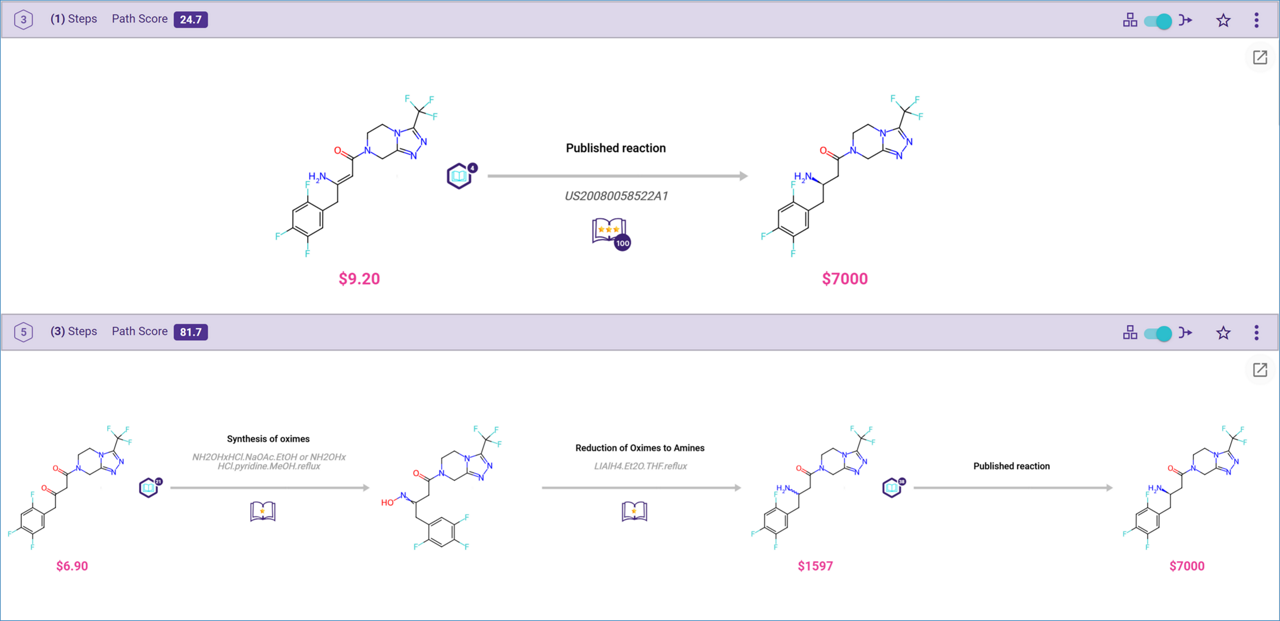
To find pathways containing published reactions or reactions suggested by machine-extracted rules, use ‘published’ or ‘machine’ as keywords in the filter.
.png)

